Mr. Rosa is an entrepreneur with three decades of experience in the medical device industry spanning a variety of technologies and products. In addition to CEO roles with early-stage medical device companies, Mr. Rosa’s background also includes senior roles with C.R. Bard Inc., Boston Scientific Inc., and St. Jude Medical, where his responsibilities included marketing, product development and business development. He has been named as an inventor on multiple medical device patents, serves on seven corporate boards, and has raised $200M in the capital markets. Mr. Rosa holds an MBA from Duquesne University and a BS in Commerce and Engineering from Drexel University.


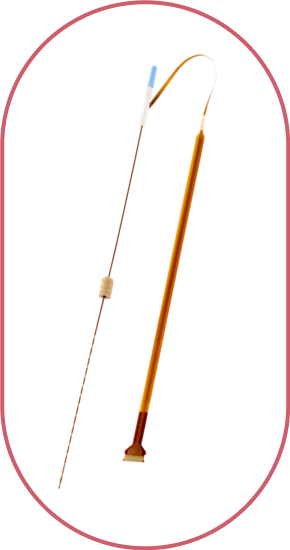 Evo® sEEG-RF
Evo® sEEG-RF 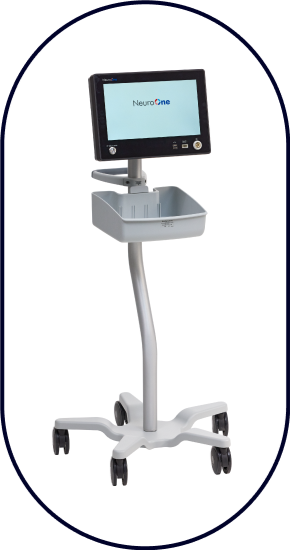 NeuroOne Radiofrequency Generator
NeuroOne Radiofrequency Generator